
Providing on-demand and customizable training solutions.

We offer on-demand training products for your team that are built to the same caliber as our award-winning, bespoke learning solutions.
Our courses serve a wide range of functions, including clinical, regulatory, manufacturing, field sales, marketing, commercial excellence, medical affairs, and home office staff. Annual updates ensure our products remain current.

ESSENTIAL SKILLS

Communicating With Confidence™
Half-day or full-day workshop | Essential Skills
Often, the root cause of miscommunication is a gap between what a person intended to communicate and how it was interpreted. In Communicating With Confidence™, we introduce a continuous communications model that shows attendees how to reduce the gap between intent and impact.

Communicating and Creating Value
Half-day workshop| Essential Skills
The process of selling has changed forever. The difference between sales professionals who thrive and those who struggle to survive is how well they harness and align the value of their products and services to customers. There is a widening gap between what companies offer and what customers actually value and are willing to pay for.

Customer Interactions in a Virtual Environment™
Half-day workshop| Essential Skills
Being an effective leader in a virtual environment is no longer a nice to have – it is a necessity. While professionals now have extensive experience operating in a virtual environment, that does not mean that they are using the optimal strategies to engage with others. Enable your team to structure and lead impactful meetings in a digital environment through Customer Interactions in a Virtual Environment™.

Customer-Focused Negotiations™
Half-day or full-day workshop | Essential Skills
Customer-Focused Negotiations™ prepares individuals for greater success negotiating. In this engaging workshop you'll gain hands-on experience with negotiation styles, tactics, and positional motivation, that will allow you to find creative and successful negotiation solutions.

Influencing With Impact™
Half-day or full-day workshop | Essential Skills
An ability to influence others and gain support for your ideas is an essential element to sustained success in every position. This applies whether you’re dealing with co-workers, managers, executives, or direct reports. Organizational structures are increasingly flatter and value chains from customers to suppliers are longer and more connected.
As a result, influencing others where you have no direct control or authority has become a vital skill…one that often goes undeveloped. This lack of skill often leads to a “whoever shouts the loudest” approach at one end of the spectrum, to a reluctance to engage at the other. With either approach, the results are less than optimal.

Key Account Management
3.5 hr e-learning and 16 hr workshop | Essential Skills
The pharmaceutical model is changing from a one-size-fits-all, generalist approach to a highly specialized service model based on key account selling. The Key Account Management program teaches you how to build and implement an account plan. It follows The Key Account Planning Process that maximizes impact with an optimal return on investments, taking into consideration the multiple stakeholders involved throughout the process.

Medical Affairs Professional Skills Workshop
~6.75 hrs | Essential Skills
Engaging and adaptable workshop that enables your MSL team to master communication tactics that increase their ability to bring value to external and internal stakeholders. Topics covered include active listening, gathering and leveraging clinical insights, and navigating difficult conversations. Each section has a framework for participants to apply in the field. The participants reflect on their skills and identify areas in which they want to develop their skills which is used at the conclusion where they develop an Action Plan.

Persuasive Presentation Skills
4 hrs | Essential Skills
Presentations are a primary opportunity to communicate value. Gaining the skills and techniques necessary to present effectively are achieved by following the techniques taught during this interactive workshop. Participants will use the 4P process and learn how to adjust their style, delivery, and duration based on the audience.

Selling Higher, Broader, and Deeper™
Half-day or full-day workshop | Essential Skills
Selling in today’s environment requires a different set of skills and strategies that focus on understanding the role of senior executives in the buying process. Individuals must not only understand the issues and concerns of their C-suite customers, but they have to think and talk like executives. This includes knowing where in the sales cycle they get involved, why they get involved, and what this means for an effective sales strategy.
SCIENCE
Acute Myelogenous Leukemia
~1.50 hrs | Oncology
This module provides a comprehensive, biannually updated overview of the acute myelogenous leukemia disease state, clinical presentation, diagnostic workup, classification & prognosis, FDA-approved treatments, and treatment recommendations.

Biotechnology
~1.00 hrs | Science
This module is an excellent foundational resource that covers: Recombinant DNA technology; Gene therapy; Immunotherapies; Hematopoietic stem cell transplants; CAR-T technology; Tumor-infiltrating lymphocyte therapy; Monoclonal antibody-based treatment; Antibody-drug conjugates; and Bispecific antibodies.
Bones, Skeleton, & Muscle
~0.25 hrs | Anatomy & Physiology
Provides an overview of the classes and structure of bones and joints; the functions of bones; the constituents of muscle tissue; and the origin, insertion, and action of major muscles.
Breast Cancer
~1.50 hrs | Oncology
This module provides a comprehensive, biannually updated overview of the breast cancer disease state, clinical presentation, diagnostic workup, classification & prognosis, FDA-approved treatments, and treatment recommendations.
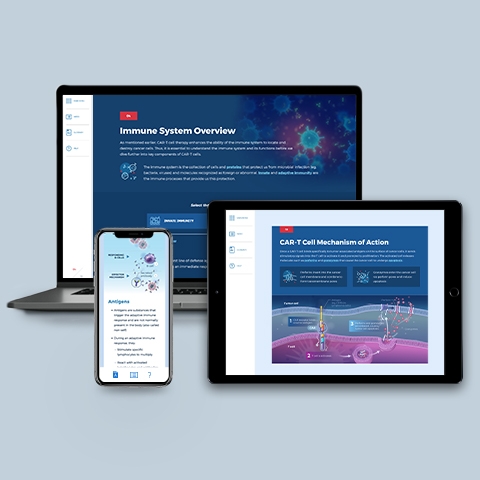
CAR-T Primer
~0.50 hrs | Science
The CAR-T module is an excellent foundational resource that describes: Human immune system cell types; CAR-T mechanism of action; Major steps in CAR-T therapy; and FDA-approved CAR-T treatments.
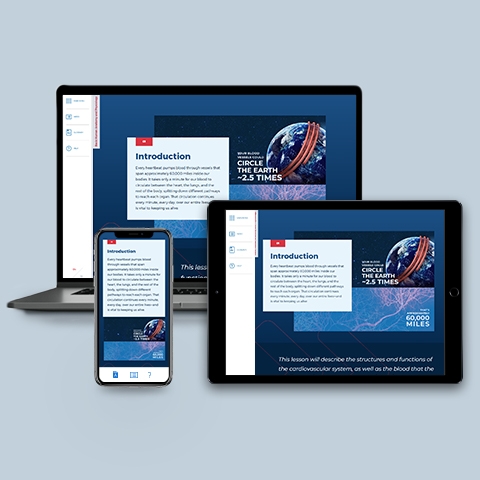
Cardiovascular System
~0.25 hrs | Anatomy & Physiology
Reviews the life-sustaining anatomy and physiology of blood, heart, and miles of blood vessels that run through our bodies.
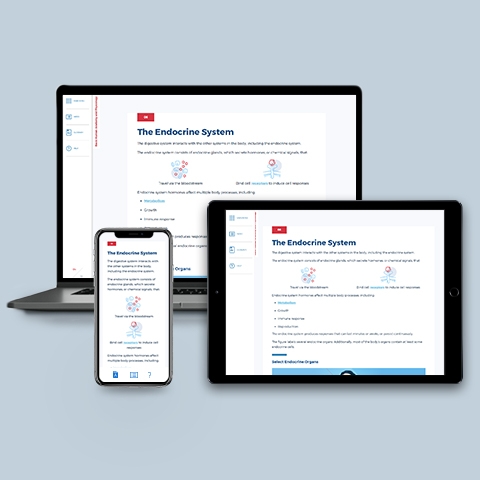
Digestive and Endocrine System
~0.25 hrs | Anatomy & Physiology
Reviews the anatomy and physiology involved in the conversion of foods into the biochemical building blocks that fuel our body's cells. This module also provides an overview of the anatomy and physiology of endocrine glands and hormones.
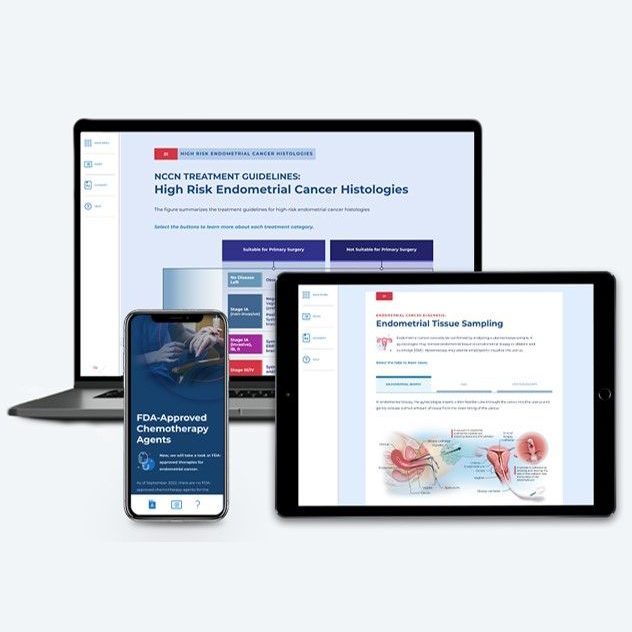
Endometrial Cancer
~1.25 hrs | Oncology
This module provides a comprehensive, biannually updated overview of the endometrial cancer disease state, clinical presentation, diagnostic workup, classification & prognosis, FDA-approved treatments, and treatment recommendations.
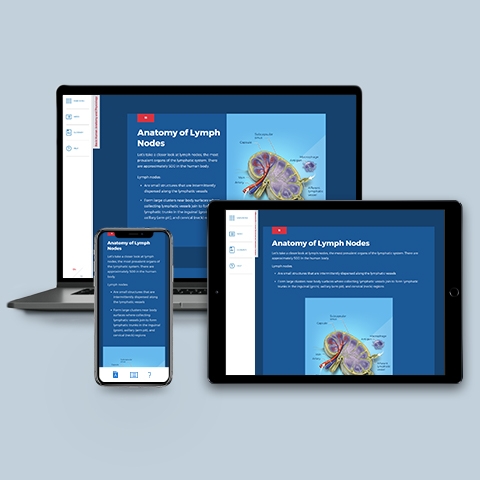
Immune & Lymphatic Systems
~0.25 hrs | Anatomy & Physiology
Describes the amazing array of defensive tactics that our bodies use to protect us from hostile bacteria, viruses, and fungi. Topics include innate, adaptive, and humoral immunity; antigens, antibodies, B-cells, and cellular immunity; and the anatomy and physiology of lymphatic vessels, lymph nodes, and spleen.
Introduction to Oncology
~1.00 hr | Oncology
This module provides you with an annually updated source of training on normal cell division and ways that disordered cell division can lead to cancer, cancer classification, cancer epidemiology, common signs and symptoms of cancer, cancer screening, diagnosis, and staging, and cancer treatments.

Medical Statistics
~1.00 hrs | Science
This module is an excellent foundational resource for customer-facing teammates who communicate clinical data to clients. It provides your team with an annually updated source of training on: Clinical study design and data; Statistical significance; Treatment effect measures; Confidence intervals; Variations in data analysis; and Data visualization

Medical Terminology
~2.00 hrs | Science
The world of medicine, like many other professions, has a language unto itself. For example, when doctors speak to each other, they use words like “polyarteritis” and “intramyocardial” instead of speaking what you might call plain English. However, these words are English, and in order to understand what you read in medical publications and have meaningful discussions with healthcare professionals, you need to understand this form of English, known as medical terminology.
Nervous System
~0.25 hrs | Anatomy & Physiology
This module describes the wonderfully complex and powerful central and peripheral nervous systems. Includes anatomy and physiology of neurons, synapses, neurotransmitters, the brain, spinal cord, sensory receptors, nerves, and axons.
Non-Small Cell Lung Cancer
~1.50 hrs | Oncology
This module provides a comprehensive, biannually updated overview of the non-small cell lung cancer disease state, clinical presentation, diagnostic workup, classification & prognosis, FDA-approved treatments, and treatment recommendations.
Ovarian Cancer
~1.50 hrs | Oncology
This module provides a comprehensive, biannually updated overview of the ovarian cancer disease state, clinical presentation, diagnostic workup, classification & prognosis, FDA-approved treatments, and treatment recommendations.
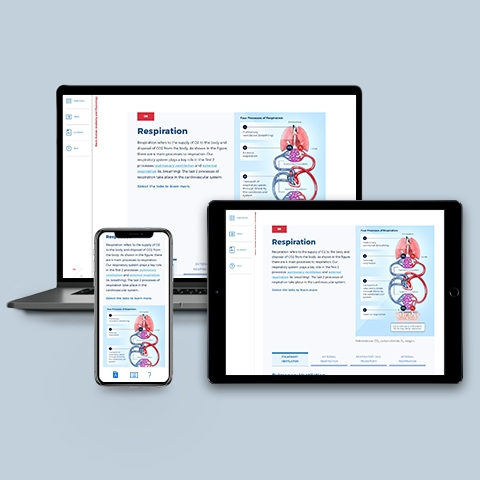
Respiratory System
~0.25 hrs | Anatomy & Physiology
This module provides an overview of the anatomy and physiology of the upper and lower respiratory systems, and the process of gas exchange between the blood, lungs, and body tissues.
MARKETPLACE

340B Program
~12 min | Market Access Academy
To increase affordability of prescription drugs for vulnerable populations, programs like the 340B Drug Pricing Program were developed. This module will build your knowledge on the 340B Drug Pricing Program, including its key features, the types of healthcare organizations eligible to participate in the program, and some potential controversies associated with the 340B Drug Pricing Program.

Claims Adjudication
~12 min | Market Access Academy
In between a doctor writing a prescription and a patient getting their medication, a pharmacy will submit an insurance claim—but then what? This module will discuss claim adjudication and explain why health plans deny claims submitted by a pharmacy and how this can affect a patient’s access to medication.

Clinics
~12 min | Market Access Academy
Where do you go when you have a broken bone or an infection? What if your primary healthcare provider isn’t available? This module will develop your understanding of hospital outpatient clinics, ambulatory surgery centers, urgent care centers, and retail clinics.

Community Pharmacies
~12 min | Market Access Academy
This module will introduce you to community pharmacies and provide examples of the varied settings for such pharmacies, their personnel, and how medication is distributed to them. Additionally, you will learn about other classifications of pharmacies.

Copay Cards
~12 min | Market Access Academy
The rising cost of prescription drugs has forced many patients to try to find ways to save money however they can, such as using copay cards. This module will introduce you to copay cards, how copay cards are used and processed, and copay adjustment programs.

Decoding Hospitals
~12 min | Market Access Academy
This module will help you gain a foundational understanding of hospitals, including their common characteristics, organizational structure, and a basic overview of their pharmacy and formulary systems.

Drug Development & Approval
~0.50 hrs
This module provides an overview of the pharmaceutical drug development process from discovery through post-marketing surveillance and discusses the regulatory requirements involved in developing, approving, and marketing drugs in the United States, Canada, European Union, Japan, and China.

Drug Purchasing, Pricing, & Distribution
~12 min | Market Access Academy
This module offers an in-depth overview of the roles various stakeholders play along a prescription drug's journey to reach the patient and the transactions that occur between these stakeholders throughout the drug purchasing, pricing, and distribution process.

EHRs and Clinical Decision-Making
~12 min | Market Access Academy
It is important for sales professionals to understand the functionality of clinical decision support tools used in major accounts because these tools suggest potential next steps for treatments, and thus can significantly impact access to your products at the point of care. This module will help you to better understand some of the functions and benefits of clinical decision support systems.
Ensuring Healthcare Quality
~12 min | Market Access Academy
This module reviews the definition of quality in US healthcare, how promoting quality is used to reform healthcare, the categories of quality measures, and prominent groups that develop US healthcare quality measures and reports.

Fundamentals of Pharmaceutical Sales
~2.00 hrs
This module consists of 5 chapters that describe how pharmaceutical products are researched, developed, promoted, an overview of managed care, and key customers for pharmaceutical sales representatives.

Healthcare Providers—Nurses
~12 min | Market Access Academy
In this module you’ll learn about the different types of nurses and the important roles they play in providing care and facilitating access to medication.

Healthcare Providers—PAs, NPs, & Others
~12 min | Market Access Academy
In this module we’ll touch on the roles of physician assistants, nurse practitioners, care coordinators/navigators, disease educators, and health coaches.

Healthcare Providers—Pharmacists
~12 min | Market Access Academy
We may not think twice about walking into a pharmacy to pick up a prescription—or about the pharmacists who make that possible. Yet these professionals check insurance coverage, often make drug recommendations, and hand the medication to the patient. These and other responsibilities position pharmacists at the front and center of market access. In this module, you will learn about the training and responsibilities of these healthcare providers.

Healthcare Providers—Physicians
~12 min | Market Access Academy
Understanding physicians and their training can help you to build better relationships with them as your customers, as they are essential to market access. This module will provide you with insight on physician training, the different categories of physicians, and their respective backgrounds and responsibilities.

Hospital Pharmacy
~12 min | Market Access Academy
This module will provide an overview of the services and staff that make up the pharmacy departments in hospitals.

Hospital Reimbursement
~12 min | Market Access Academy
When a patient is treated at a hospital, the hospital will be compensated by third-party payers for services provided to the patient. This module provides an in-depth discussion of the several types of hospital payers and reimbursement arrangements along with cost-reducing reimbursement strategies used by payers.
IDN/ACO Account Opportunities
~12 min | Market Access Academy
Integrated delivery networks (IDNs) and accountable care organizations (ACOs) are big, sometimes huge organizations. In this module, you will learn about IDN/ACO priorities and opportunities for you and your team to collaborate on IDN/ACO account management.

Integrated Delivery Networks (IDNs)
~12 min | Market Access Academy
As the US healthcare system undergoes major changes with a shift from volume to value, integrated delivery networks (IDNs) and other large healthcare networks are taking center stage. This module will help you understand how IDNs are structured to streamline care.

Introduction to Commercial Payers
~12 min | Market Access Academy
Knowing the meaning behind the term "payer" is crucial to understand the healthcare system. In this module, you will become familiar with what a payer is, the different types of payers in the commercial healthcare setting, and the key stakeholders along the commercial payer continuum.

Introduction to Managed Care
~12 min | Market Access Academy
This module will help you to understand some of the different types of managed care entities and key stakeholders along the managed care continuum.

Introduction to Public Payers
~12 min | Market Access Academy
Tens of millions of Americans receive healthcare coverage through a public health plan. In this module, you will be introduced to common public health plans offered within the US along with their respective characteristics.

Key IDN Stakeholders
~12 min | Market Access Academy
In this module, you will learn about the roles, responsibilities, and priorities of some key stakeholders within an IDN who may play an important role in your sales strategy and market access to your products.

Nonclinical Hospital Stakeholders
~12 min | Market Access Academy
This module will describe the roles of hospital purchasing agents, case managers, discharge planners, and health information managers.

P&T Committees and Formularies
~12 min | Market Access Academy
This module will help you gain a broad understanding of who determines which drugs to include on drug formularies along with how formularies are structured.

Patient-Centered Medical Homes
~12 min | Market Access Academy
This module will guide you in gaining a foundational understanding of patient-centered medical homes, including core beliefs that drive the patient-centered medical home model and the various team members that contribute to the model's success.

Payer Mix
~12 min | Market Access Academy
Coverage of an individual's health services and prescription drugs will differ depending on whether they are covered by a public or commercial payer. These differences trickle down and impact other healthcare stakeholders, such as healthcare providers. This module aims to educate you on payer mix, how payer mix may affect a healthcare provider's finances, and the impacts payers have on drug prescriptions.

Paying for Healthcare: Terms to Live By
~12 min | Market Access Academy
Before you engage with complex US healthcare payer and reimbursement concepts, you will need to be familiar with some key terms. In this module you will learn about key healthcare payer and reimbursement terminology that will provide a solid foundation for more advanced training.

Post-acute & Long-term Care Facilities
~12 min | Market Access Academy
In this module, you will be introduced to post-acute and long-term care and the facilities that provide such healthcare services.

Prior Authorization
~12 min | Market Access Academy
With the help of this module, you will familiarize yourself with the prior authorization process and where payers and physicians may be at odds when it comes to prior authorization.

Profiling a Hospital Account
~12 min | Market Access Academy
This module discusses the characteristics and operational factors that can help you develop a financial profile for a hospital.

Reducing Healthcare Costs
~12 min | Market Access Academy
As a result of increased national health spending, market access to medical services and prescription drugs has become increasingly challenging. One of the ways healthcare payers attempt to combat increased spending is through benefit design. In this module, you will learn about some ways that payers use benefit design to control the cost of medical services and prescription drugs.

Reimbursement Models
~12 min | Market Access Academy
Payer reimbursement for a physician's services is determined based on various factors that translate into several types of reimbursement models. In this module, you will learn about these reimbursement models and their respective characteristics.

Specialty Pharmacies
~12 min | Market Access Academy
In this module, you will learn about specialty pharmacies and the reimbursement logistics and distribution models involved in providing patients with access to specialty medications.

The Essential Role of Health Information Technology
~12 min | Market Access Academy
Electronic health data is a vital component of proper patient care in today's healthcare environment. This module provides a discussion of health information technology and the role it plays in enhancing the quality and value of healthcare.

The Impact of Health Information Technology on Hospital Prescribing
~12 min | Market Access Academy
Health information technology has grown into a fundamental aspect of the healthcare landscape, managing information that might otherwise overwhelm healthcare providers. This module will describe the health information technologies that assist providers with their prescription and treatment decisions.

Types of Hospitals
~12 min | Market Access Academy
This module will help you recognize the differences between types of hospitals based on their ownerships, funding structures, and the types of patients they treat.

Understanding Accountable Care Organizations (ACOs)
~12 min | Market Access Academy
One of the goals of the Affordable Care Act (ACA) was to reduce Medicare costs. To help accomplish this, the ACA encouraged providers to form accountable care organizations (ACOs) and adopt value-based reimbursement of healthcare. In this module, you will learn about the different types of ACOs in the marketplace and how they operate.

Understanding Population Health
~12 min | Market Access Academy
In response to US health spending being primarily allocated toward medical care, population health has emerged as an approach that brings nonmedical determinants of health into focus. This module takes a deeper dive into population health and the major building blocks associated with its implementation.

Who’s Who in the Physician Practice
~12 min | Market Access Academy
To maximize effectiveness, sales representatives must become familiar with the roles at a physician’s practice. This module will discuss the staff commonly working at a physician’s practice and how they might interact with sales representatives.
GxP/COMPLIANCE

Animal Care Requirements
~0.50 hrs | GLP - Nonclinical Operations & Process
This module will cover a broad range of topics related to animal care in GLP studies. This includes governing bodies who regulate animal care in testing, requirements for living conditions for animals in a GLP environment, animal identification expectations, and other important concerns.

Annual GCP Refresher
~0.50 hrs | GCP | 🛒
The GCP Refresher module will cover the top 10 regulatory observations from the US Food and Drug Administration (FDA), updates to ICH GCP and global regulations, and employ a case study.

Annual GLP Refresher
~0.50 hrs | GLP - Annual GLP Refresher
The GLP Refresher module will cover the top 10 regulatory observations from the US Food and Drug Administration (FDA) and other global health authorities and updates to global regulations and guidance. You will also engage in a case study.

Annual GMP Refresher
~0.50 hrs | GMP - Annual GMP Refresher
The GMP Refresher module will cover the top 10 regulatory observations from the US Food and Drug Administration (FDA) and other global health authorities (HA) and updates to global regulations and guidance. You will also engage in a case study.

Annual Product Reviews & Regulatory Submission Updates
~0.50 hrs | GMP - Quality Systems
Every year pharmaceutical manufacturers must conduct an annual product review (APR). This module defines what should be included in an APR and the different types of regulatory change submission updates required by the FDA.

Authoring & Implementing GMP-Compliant SOPs
~0.50 hrs | GMP - Quality Systems
In this module, you will learn the key elements of SOP identification, authoring, change control, and improvement. You will learn the full journey of an SOP and receive an interactive view of “best practices” for SOP development.

Batch Records & Batch Record Review
~0.50 hrs | GMP - Quality Systems
The details documented in batch production records provide a complete picture of the manufacturing and testing processes. As a result, batch records are the most highly viewed records in regulatory inspections and audits. This module will explore batch record content, requirements, and review mechanisms to ensure compliance with global mandates.

Best Practices for CMO/CDMO Vendor Selection, Qualification, Contracting, & Oversight
~0.50 hrs | GMP - Manufacturing Controls
This module describes best practices for vendor selection, how to qualify selected vendors, and how quality agreements protect the interests of all parties by clearly defining CGMP-related roles and responsibilities for manufacturing products within a quality system.

CRO Oversight Best Practices
~0.50 hrs | GCP - Clinical Operations & Process 🛒
In the life sciences, sponsor companies are always ultimately responsible for the management and compliance of their CROs when conducting clinical studies. This module will provide you with instruction on the applicable regulations and guidance for CRO oversight, outline the necessary steps and process, and provide best practices.

CRO Qualification, Selection, & Monitoring
~0.50 hrs | GCP - Clinical Operations & Process
This module will present a step-by-step overview of the required and critical elements of CRO selection, qualification, and procurement. This interactive training will also provide best practices for maintaining solid relationships with CROs.

Change Management
~0.50 hrs | GMP - Quality Systems
ICH Q10 describes change control as a “systematic approach to proposing, evaluating, approving, implementing, and reviewing changes.” This module will present an interactive overview of what change control is, why it’s important, how it should be implemented, and measuring success.

Characterization and Use of Test & Control Articles, Reagents, and Solutions
~0.50 hrs | GLP - Nonclinical Operations & Process
This course will outline global regulations and guidance for the characterization and use of test and control articles.

Clinical Audits & Inspections
~0.50 hrs | GCP - Deviation Investigations, RCA, CAPAs, & Audit Preparation
This on-demand module will help you understand what an audit is, what to do to prepare for an inspection, and how to appropriately respond to resulting observations.

Clinical Quality Management, Risk Management & Sponsor Responsibilities
~0.50 hrs | GCP - Clinical Operations & Process
This module will focus on the sponsor’s responsibilities concerning the quality risk management of a clinical trial while offering strategies to avoid risk and improve outcomes.

Clinical Responsibilities for Regulatory Submission & Compliance
~0.50 hrs | GCP - Regulations, Guidance, & Submissions
This module is an overview of the roles and responsibilities the clinical team has in ensuring compliance for key deliverables that will need to be reported in regulatory submissions.

Clinical Trials: Adverse Events
~0.50 hrs | GCP - Clinical Operations & Process
In this module, you will receive an overview of the types of Adverse Events (AE), how to report them, where to report them, and how to establish an AE reporting system.

Clinical Trials: Roles & Responsibilities
~0.50 hrs | GCP - Clinical Operations & Process
This module will review the functional and specific roles necessary to conduct a clinical study. Content will move you through a discussion of functional roles (sponsor, sites, IRBs, vendors, etc.) and expand on the specific vocations for each function.

Comparison of the FDA, EPA, & OECD GLPs
~0.50 hrs | GLP - Regulations and Guidance Documents
This course will provide you with an overview of the global requirements and guidance for GLP compliance, plus key similarities and differences. The content will provide guidance on best practices for compliance.

Contract Laboratory Qualification and Contracting
~0.50 hrs | GLP - Nonclinical Operations & Process
This module will discuss considerations for qualifying and selecting a contract laboratory, the procurement process, and defining the necessary elements of a quality agreement to achieve the goal of completing a successful GLP study.

Corrective & Preventive Actions: Planning, Implementing, & Measuring Effectiveness
~0.50 hrs | GCP - Deviation Investigations, RCA, CAPAs, & Audit Preparation
Learners will be taken on a deep dive into the steps needed to identify and implement a robust CAPA. Additionally, participants can expect detailed instruction on measuring CAPA success and tracking progress.

Data Integrity & Good Documentation Practices in the GLP-Compliant Lab
~0.50 hrs | GLP - Documents and Data Integrity
This module will help you understand the critical nature of accurate, contemporaneous, and quality data in GLP studies. You will review what data integrity is and how to achieve it, plus learn the relationship between data integrity and GDocP in a GLP environment.

Data Integrity Controls: Computer System Validation
~0.50 hrs | GMP - Quality Systems
Manufacturing software and hardware systems must go through a process called computer system validation (CSV) to ensure that the systems function as intended, and staff are trained to use them properly. This module will discuss requirements and best practices for data integrity and CSV in a life sciences manufacturing environment.

Data Integrity Controls: Quality Control Out-of-Specification Investigations
~0.50 hrs | GMP - Quality Systems
Management of out-of-specification (OOS) results is a critical factor to ensure data integrity and is often cited during inspections. This module will provide you with a foundational understanding of how to establish data integrity controls and investigate OOS issues.

Designing & Conducting a Clinical Trial
~0.50 hrs | GCP - Clinical Operations & Process
The Designing & Conducting a Clinical Trial module will provide you with a detailed overview of how trials are conducted, the different phases of a clinical trial, and the roles/functions needed to carry out a clinical study.

Deviation Investigations, Root Cause Analysis, & CAPA
~0.50 hrs | GCP - Deviation Investigations, RCA, CAPAs, & Audit Preparation
By the end of this module you will be able to identify deviations within clinical trials, how to investigate them, and the methods used to correct and prevent them.

Deviation Investigations, Root Cause Analysis, & CAPA Overview
~0.50 hrs | GMP - Quality Systems
This module will cover regulatory expectations and standard processes for successful deviation investigations. It will also provide an overview of RCA tools and creating, implementing, and reviewing the efficacy of successful CAPAs.

Deviation Investigations, Root Cause Analysis, and Corrective & Preventive Action
~0.50 hrs | GLP - Deviations
In this module, you will receive an overview of deviation types and when they require investigation, and different types of RCA tools to identify root causes. Finally, you will learn how to design and implement effective CAPA strategies.

Documenting the GLP study: From Study Protocol to Final Report
~0.50 hrs | GLP - Documents and Data Integrity
This module will outline the requirements for capturing and documenting data in a GLP study. Additionally, the content will provide a foundational understanding of GLP record types and the required elements of a final report. You will also learn how GDocP plays an important role in data capture and reporting.

GCP Regulations, Guidance & Informed Consent
~0.50 hrs | GCP - Regulations, Guidance, & Submissions
This module provides a summary of FDA and EU regulations and ICH guidance for good clinical practice.

GDPR Requirements for Clinical Research Data
0.50 hrs | GDPR
This module will explore a range of topics that explain how GDPR applies to the processing and protection of clinical research data, including the lawful bases for processing personal data within the confines of a trial, related safeguards, clinical data autonomy under GDPR, and the balance between GDPR and HIPPA requirements.

GDPR Requirements for Employee Data
0.25 hrs | GDPR
This module will discuss how the General Data Protection Regulation (GDPR) is applied to employee data and affects the employer-employee relationship.

GDPR Requirements for Marketing Data
0.50 hrs | GDPR
This module will explore a range of topics that explain how the European Union General Data Protection Regulation, or GDPR, applies to the types of personal data marketing companies typically collect from consumers.

GLP Inspections
~0.50 hrs | GLP - Audits and Inspections
This module will cover the roles and responsibilities of personnel during a GLP inspection, with special focus on the quality assurance unit (QAU). The content will cover internal audits, vendor audits, and health authority inspections. Additionally, you will learn about proper response activities for internal/vendor audits and inspections.

GLP Requirements for Equipment
~0.50 hrs | GLP - Nonclinical Operations & Process
By the end of this training, you will have a better understanding of the GLP equipment types, use, and regulatory mandates for qualification and validation. You will also learn about equipment maintenance and general record-keeping requirements.

GLP Requirements for the Facility
~0.50 hrs | GLP - Nonclinical Operations & Process
This course will outline the requirements for lab operations, testing facilities, waste disposal, pest control, and environmental monitoring. It will also cover specific animal care requirements related to the facility, as well as computerized systems and general security concerns.

GMP Audits & Inspections: Internal Auditing
~0.50 hrs | GMP - Audits & Inspections
This module will take a deep dive into devising and implementing solid internal audit programs that mitigate risk and foster a culture of compliance.

GMP Audits & Inspections: Responding to Health Authority & Internal Audit Observations
~0.50 hrs | GMP - Audits & Inspections
It is one thing to be prepared for an inspection or internal audit, but responding to subsequent observations is an entirely different matter. This module will focus on responding to feedback from a health authority or an internal audit observation.

GMP Inspections: Preparing for & Managing Health Authority Inspections
~0.50 hrs | GMP - Audits & Inspections
In this interactive module, you will receive guidance on how best prepare for and conduct a successful GMP inspection. Content will take you through preparing your staff, facilities, documentation, and processes to ensure successful outcomes.

GMP Management Responsibilities
~0.50 hrs | GMP - Quality Management
This module will take a deep dive into management responsibilities as dictated by global regulations and ICH Q10.

General Data Protection Regulation & Information Systems
0.25 hrs | GDPR
During this training, we will look at the methods and systems used by organizations to comply with GDPR and how data within information systems is handled.

Global Regulatory & Compliance Overview
~0.50 hrs | GMP - Global Regulations & Compliance
This course will provide you with a detailed overview of the requirements for manufacturing and quality control as outlined by major global health authorities (HA). Content will also cover standards organizations, industry cooperatives, and global guidance. This module will also take you through key differences–and similarities–between specific requirements of each HA.

Global Regulatory Landscape: Key Differences
~0.50 hrs | GCP - Regulations, Guidance, & Submissions
This module provides an overview of the regulatory differences and requirements for conducting clinical trials for medicinal products in human participants in the United States, Europe, Canada, China, and Japan.

Good Documentation Practices & Introduction to Data Integrity
~0.50 hrs | GMP - Quality Management
Good Documentation Practices (GDocP) are a critical portion of GMP and the manufacture of therapeutic products. Inclusive of data capture, data outputs, change control, access, and archiving. This module will give you an overview of GDocP and data integrity in a GMP environment.

Good Documentation Practices in a Clinical Environment
~0.50 hrs | GCP - Documents & Data Integrity
Good Documentation Practices (GDocP) are a critical portion of the clinical trial process, inclusive of document authoring, change control, access, and archiving. The Good Documentation Practices in a Clinical Environment module summarizes standard practices for good clinical documentation.

ICH Q8/Q9/Q10 Deep Dive
~0.50 hrs | GMP - Quality Management
This module will provide details about the ICH guidance documents that have been adopted to define a pharmaceutical quality system (PQS). These documents encompass a modernized approach to product development, address expectations for a quality risk management process, and define a quality system framework for overseeing risk management performance.
Introduction to GDPR
~0.50 hrs | GDPR
This module will take roughly 1 hour to complete and provides a high-level overview of General Data Protection Regulation (GDPR). It spans across critical areas that define GDPR, providing an overview of what GDPR is, roles of regulators and responsible personnel, and associated privacy rights.

Introduction to Good Clinical Practices
~0.50 hrs | GCP - Clinical Operations & Process
The Introduction to Good Clinical Practices module provides an overview of the rules, regulations, and guidelines that companies must or should follow during clinical trials to ensure the protection of human participants.
Introduction to Good Laboratory Practices
~0.50 hrs | GLP - Nonclinical Operations & Process
This module provides an overview of the rules, regulations, and guidelines that companies must or should follow during nonclinical studies to show the efficacy of nonclinical studies, and why these rules are important.

Introduction to Good Manufacturing Practices
~0.50 hrs | GMP - Nonclinical Operations & Process
The Introduction to Good Manufacturing Practices module provides an overview of the rules, regulations, and guidelines that companies must or should follow during manufacturing to ensure the quality and safety of products.
Introduction to GxP
~0.50 hrs | GLP - Nonclinical Operations & Process
The Introduction to GxP module provides a summary of the rules, regulations, and guidelines that companies must follow when they research, manufacture, and market therapeutic products.
Introduction to GxP
~0.50 hrs | GCP - Clinical Operations & Process
The Introduction to GxP module provides a summary of the rules, regulations, and guidelines that companies must follow when they research, manufacture, and market therapeutic products.
Introduction to GxP
~0.50 hrs | GMP - Nonclinical Operations & Process
The Introduction to GxP module provides a summary of the rules, regulations, and guidelines that companies must follow when they research, manufacture, and market therapeutic products.

Introduction to Health Authority Inspections & Common Inspection Findings
~0.50 hrs | GMP - Audits & Inspections
In the highly regulated life sciences industry, good manufacturing practice (or GMP) regulatory agency inspections are a fact of life. This module will provide an overview of the inspection process, critical roles, best practices to prepare, and common inspection findings to give you perspective on where others commonly fail.

Investigator Responsibilities & IRB Review
~0.50 hrs | GCP - Clinical Operations & Process 🛒
By the end of this training, you will have an understanding of the roles and responsibilities of investigators, delegates, and the Institutional Review Board (IRB) in ensuring compliance with regulatory guidances and human participants’ protection in clinical investigations.

Managing Data Integrity and the Electronic Record Life Cycle
~0.50 hrs | GCP - Documents & Data Integrity
The integrity of data and documents is a critical component to running a clinical study. In this module, you will learn how to ensure your data is accurate and secure, and review key components of document and data storage and change control. Finally, you will learn about the different types of data, documents, systems, and how they all tie in to study submissions.

Managing Product Complaints, Field Alerts, & Recalls
~0.50 hrs | GMP - Quality Systems
This module explores all the actions a manufacturer must take to manage the receipt, investigation, and reporting of complaints, which includes field alert reports (FARs) and product recalls.

Microbial Control: Cleaning Validation & Personnel Practices
~0.50 hrs | GMP - Manufacturing Controls
This module will examine the regulatory agency expectations that ensure the appropriate performance of microbial control processes. This includes cleaning, cleaning validation, and personnel practices.
Microbial Control: Facility Design, Water, & Air Quality
~0.50 hrs | GMP - Manufacturing Controls
This module is a deep dive into health authority expectations and industry best practices for facility construction and design, water systems, and environmental monitoring practices to mitigate risk of microbial contamination of manufactured products.
Monitoring & Quality Risk Management
~0.50 hrs | GCP - Clinical Operations & Process
This interactive module will give you the necessary background to identify key elements of a risk-based monitoring plan. Content will instruct you on how to implement plans and make continuous improvements.

Organization & Personnel in GLP Studies
~0.50 hrs | GLP - Nonclinical Operations & Process
By the end of this training, you will have a clear picture of specific key roles within a nonclinical GLP study. This includes functional roles within a study and the key organizational players.

Process Validation & Media Fills
~0.50 hrs | GMP - Manufacturing Controls
The Process Validation and Media Fills module will take you through a step-by-step overview of validating manufacturing processes through the use of process simulations, more commonly referred to as “media fills.”

Quality Systems Basics & Expectations of Personnel
~0.50 hrs | GMP - Quality Systems
"Quality Systems" are the documented procedures, processes, and systems used to ensure the safe and repeatable manufacture of the therapeutic process. This module will outline the requirements and guidance for quality systems in a GMP environment.

Risk Management & Continuous Improvement in a GMP Environment
~0.50 hrs | GMP - Quality Systems
Quality risk management (QRM) is a “systematic process for the assessment, control, communication, and review of risks to the quality of the drug product across the product life cycle.” This module answers the questions of when and how QRM is used and describes the entire QRM process.

Roles & Responsibilities of the Quality Control Unit
~0.50 hrs | GMP - Quality Management
By the end of this training, you will have a better understanding of the GMP activities that support the quality control unit (QCU), sometimes referred to as the quality unit (QU). This includes the components of the QCU, roles and regulatory responsibilities, and best practices for continuous improvement.

Root Cause Analysis & CAPA Deep Dive
~0.50 hrs | GMP - Quality Systems
This module will take a deeper dive into the processes, skills, and tools for conducting RCA and moving through the CAPA process. Different types of root causes will be discussed along with two models that are useful for investigating root cause.

Root Cause Analysis & Introduction to the Corrective & Preventive Actions Process
~0.50 hrs | GCP - Deviation Investigations, RCA, CAPAs, & Audit Preparation
This module will take you through deviation identification, the investigation process (inclusive of investigational root cause analysis tools), and the initial steps for CAPA creation.

Sterile Manufacturing & Key Challenges
~0.50 hrs | GMP - Manufacturing Controls
Many of the therapeutic products life sciences companies bring to market require sterility. This is especially true of complex biologics, injectables, and implantable medical devices. This module provides an overview of the requirements for facilities, management, and personnel.

Training Requirements for GLP Studies
~0.50 hrs | GLP - Nonclinical Operations & Process
In this module, you will learn the responsibilities of study personnel and management, and the special responsibilities of the quality assurance unit (QAU). Once this foundation is set, you will learn the specific training requirements for these roles and the types of training employed.
Validation & Change Control Overview
~0.50 hrs | GMP - Quality Systems
In this module, you will learn about the role of process validation and change control, and how these activities allow manufacturers to ensure they have the correct processes in place to ensure the consistent, repeatable manufacture of safe products.

Virtual Scenario: Practice Inspection
~0.50 hrs | GCP - Practice Scenarios
The Practice Inspection module will take the learner through an interactive journey through a “mock” inspection, using a scenario and questions to determine the best course of action. A “refresher” is offered at the beginning to help learners address the scenario, while feedback is offered throughout the exercise.

Virtual Scenario: Practice Deviation Investigation & CAPA Creation
~0.50 hrs | GMP - Quality Systems
The Practice Deviation Investigation and CAPA Creation module will take the learner through an interactive journey through a series of scenarios focusing on deviation investigations, CAPA identification/creation, and follow-up.

Virtual Scenario: Practice Deviation Investigation & CAPA Creation
~0.50 hrs | GCP - Practice Scenarios
The Practice Deviation Investigation & CAPA Creation module will take the learner through an interactive journey through a series of scenarios focusing on deviation investigations, CAPA identification/creation, and follow-up.

Virtual Scenario: Practice Inspection
~0.50 hrs | GMP - Audits & Inspections
This virtual practice inspection will take the learner through a series of scenarios that focus on the actions, activities, roles, and responsibilities during a regulatory agency inspection process.

Writing Effective SOPs in the GLP-Compliant Laboratory
~0.50 hrs | GLP - Nonclinical Operations & Process
In this module, you will learn the key elements of SOP identification, authoring, change control, and improvement. You will learn the full journey of an SOP and receive an interactive view of best practices for SOP development.

Writing GCP-Compliant SOPs
~0.50 hrs | GCP - Clinical Operations & Process
Writing standard operating procedures (SOPs) is a required function of all clinical studies. This module presents a unique role-play approach to help you learn key elements of SOP identification, authoring, and improvement. You will learn the full journey of an SOP and receive an interactive view of “best practices” for SOP development.